Research
Our research is focused on understanding how organs take shape during embryonic development. Current projects in the lab are addressing fundamental questions about mechanisms underlying organ formation:
1. What mechanisms establish left-right asymmetry in developing organs?
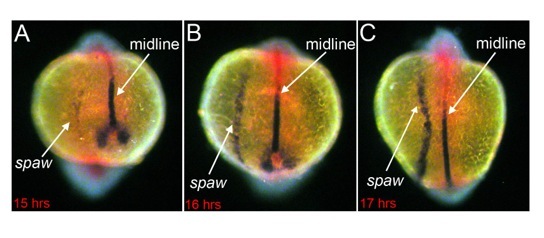
Fig. 2. Asymmetric gene expression. In the zebrafish embryo, asymmetric expression of the nodal-related gene southpaw (spaw) is initiated in left lateral plate mesoderm cells (A). Left-sided expression expands anteriorly towards developing organs (B-C). The embryonic midline provides a barrier that prevents spaw from expanding to the right side of the embryo. Embryos in A, B and C were fixed ~15, 16 and 17 hours post-fertilization, respectively and stained by RNA in situ hybridization for expression of spaw in lateral plate mesoderm and lefty1 (purple) and no tail (red) in the midline.
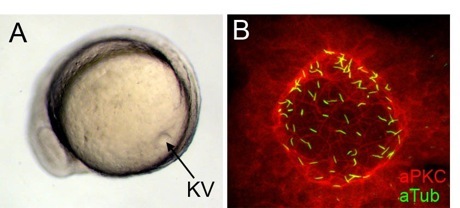
Fig. 3. Kupffer’s vesicle is a ciliated organ in zebrafish. During somite stages of zebrafish development, Kupffer’s vesicle (KV) forms in the tail region (A). Fluorescent immunostaining of KV cells (B) with atypical protein kinase C antibodies (red) to mark epithelial cells and acetylated tubulin antibodies (green) to label cilia. Each KV cell has a single cilium.
In all vertebrates, several internal organs develop conserved left-right asymmetries. For example, during heart development the initially symmetric heart tube undergoes rightward looping to establish the relative positions of chambers, septa and vascular connections (Fig. 1). Perturbation of left-right asymmetries often leads to disease--primarily congenital heart defects that can include atrioventricular septal defects, double outlet right ventricle, transposition of the great arteries and aortic arch anomalies. Our goal is to identify genes and mechanisms that advance our understanding of how organ asymmetry is established and provide candidate genes for human malformations.
Prior to asymmetric development of organs, the first break in symmetry in the embryo is at the molecular level. A conserved Nodal signaling cascade is asymmetrically initiated on the left side of vertebrate embryos (Fig. 2). This global asymmetric patterning of the embryo is thought to convey left-right information to developing organs.
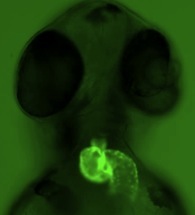
Fig. 1. Cardiac left-right asymmetry. In all vertebrates, the heart loops to the right during early stages of development. Asymmetric cardiac looping is easily visualized in transgenic zebrafish embryos that express green fluorescent proteins specifically in the heart. See also Movie 1. L=left; R=right.

R L
L R
Evidence from several species (human, mouse, rabbit, frog and zebrafish) indicates that cilia-driven fluid flow is a conserved mechanism that directs left-sided asymmetric gene expression. We have shown in zebrafish that a specialized group of motile cilia found in a transient embryonic organ called Kupffer’s vesicle (Fig. 3) generate directional fluid flow (see Movies 2 and 3) that is required for normal left-right patterning throughout the embryo. Several ongoing projects are aimed at understanding how ciliated cells in Kupffer’s vesicle are organized into a functional unit capable of generating coordinated fluid flow and left-right signals.
The mechanisms used by organs to receive and respond to left-right asymmetric gene expression remain largely unknown. The zebrafish embryo provides an excellent system to study asymmetric organ morphogenesis. We are using live imaging approaches to study how cell behaviors such as shape changes, proliferation, apoptosis or migration may impact organogenesis.
2. What pathways regulate development of ciliated organs?
In addition to regulating left-right asymmetry, motile cilia play other critical roles during embryonic development and throughout adult life. In several organs, motile cilia beat in a coordinated fashion to move fluids. Examples include respiratory epithelial cilia moving mucus through airways or ependymal cilia moving cerebrospinal fluid through the brain and spinal cord. The zebrafish embryo provides an excellent model system to study development of organs with motile cilia. In addition to Kupffer’s vesicle (discussed above), other ciliated organs form within the first 24 hours of development--including the pronephric ducts of the kidney, the otic vesicles that give rise to the inner ear (Fig. 4) and the ependymal lining of the spinal cord (Movie 4).

Fig. 4. Ciliated organs in the zebrafish embryo. By 24 hours post-fertilization (hpf) several ciliated organs have formed in the zebrafish embryo, including Kupffer’s vesicle (KV), otic vesicles (OV) and pronephric ducts (PND). Diagram of early zebrafish development modified from (Kimmel, et al. 1995).
We are using these ciliated organs in zebrafish embryo to study genes that may underlie cilia defects in humans that result in a broad spectrum of disorders collectively known as ciliopathies. Taking a candidate gene approach, we have identified several genes that control the form and function of ciliated organs. Some of these impact formation of the epithelial structure of the organ, whereas others are involved in formation of motile cilia. Using reverse genetics we are using these genes as foundations to build novel pathways that regulate development of ciliated organs.
3. What biophysical interactions drive organ formation?
Organ development depends cell shape changes that are governed by mechanical forces and tensions. Biochemical signaling pathways have been identified that regulate cell shape changes, but how these pathways interact with mechanical forces in the embryo remains poorly understood. We are collaborating with Dr. M. Lisa Manning’s group in the Department of Physics at Syracuse University to combine high-resolution in vivo imaging (see Movie 5) and molecular biology tools with image analysis and mechanical modeling techniques from physics to study interactions between signaling and forces that control organ formation. For example, Dr. Manning has generated models of cell shape changes during the development of Kupffer’s vesicle (Fig. 5) that makes predictions about forces between cells that are necessary for proper organ development.
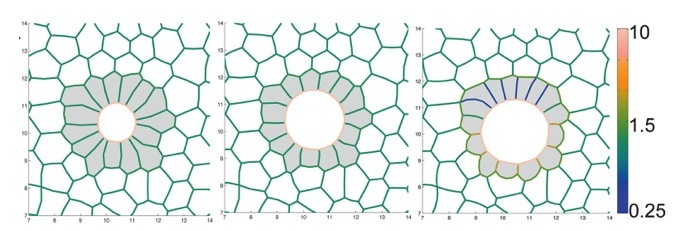
Fig. 5. Mechanical model of Kupffer’s vesicle development. Snapshots from a simulation of Kupffer’s vesicle (shaded gray) development. The color range indicates magnitude of interfacial tension between cells. This figure is modified from (Wang, et al. 2012).
In addition to modeling cell-cell interactions, we are using image analysis techniques to study tissue-tissue interactions. Time-lapse images of cells in the developing zebrafish embryo can be analyzed using particle image velocimetry to study tissue movements (Fig. 6). Our goal is to understand how external forces generated by neighboring tissues impact cell shapes in developing organs.
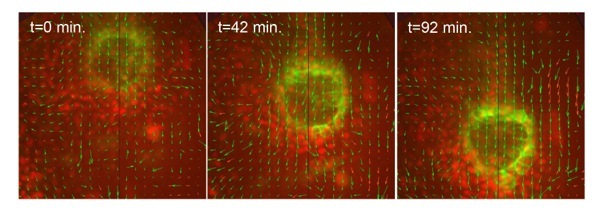
Fig. 6. Cellular flows in development. Particle image velocimetry indicates the movements of cells and tissues in a live zebrafish embryo.

